Cardiac Output Monitor Review
About C-COM
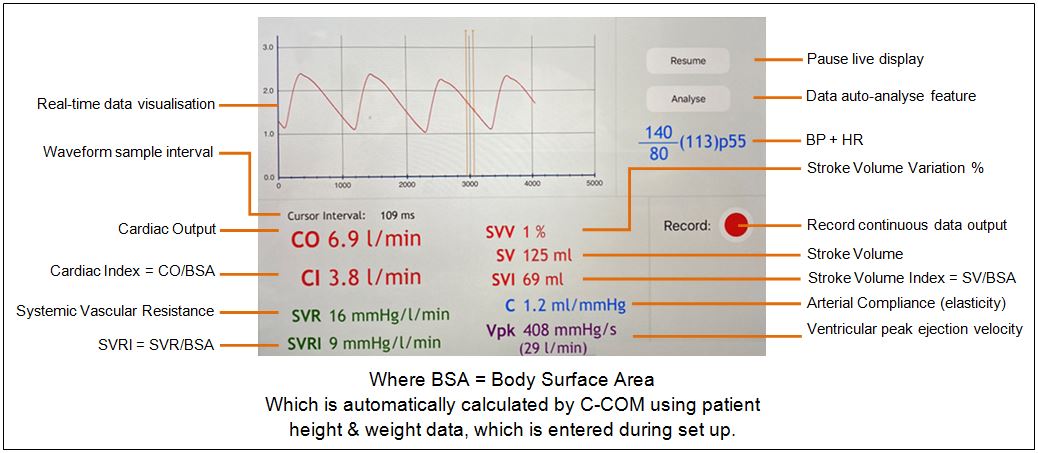
Developed on behalf of renowned Australian anaesthetist, Dr Duncan Campbell, C-COM is a proof of concept non-invasive MedTech device designed to measure a patient's cardiac output.
Project Summary
Amatek was tasked with reviewing the efficacy of the proof of concept and developing a commercialisation plan to take the product to market
The project included:
- Hardware and software review.
- Cardio-vascular mathematical model evaluation and simulations.
- Redesign estimates for hardware and software.
- Verification and validation estimates.
- Compliance programs in accordance with ISO13485 and ISO60601.
- Presentations to potential funding parties.
Outcomes
An opportunity for third parties to fund this unique project exists.